Citric Acid Ionic or Covalent
Gallic acid 8 Nucleic acids 5 Zwitterions 3 Chemical. When water is added the.
Then we dissolve and vigorously mix 08 g of PEG-NHS in 8 ml of phosphate-citric acid buffer solution which has a pH of 58 and an ionic strength of 01 M.
. The acid dissociation constant for an acid is a direct consequence of the underlying thermodynamics of the dissociation reaction. Viscosity of Binary Aqueous Solutions Involving Malonic Maleic Malic Tartaric and Citric Acids in the Temperature Range between 303 and 363 K. You have arrived exactly at the right place.
What are acids base and salt. Tremendous efforts are being made to develop electrode materials electrolytes and separators for energy storage devices to meet the needs of emerging technologies such as electric vehicles decarbonized electricity and electrochemical energy storage. Pure acids without water are composed of covalent small molecules.
It is also used. Although acetic acid is a covalent compound the hydrogen atom in the carboxyl group -COOH can go under ionization to form hydrogen cation and acetate anion. Lewis adduct is the name given to the resultant chemical.
Lemon juice contains citric acid and tamarind contains tartaric acid. What represents a formula for a chemical compound. A Strength of forces.
Ionic Chemical Bonds II. As the term describes itself general engineering is the branch of science and technology that deals with many areas of science such as electrical mechanical chemical architectural civil and. Protons are also special with respect to electron transport.
The value of the pK a changes with temperature and can be understood qualitatively based on Le Châteliers principle. A process that take place when an ionic compound dissolves in water allowing the ions in the compound to separate. Which of the following statements about enzymes are true.
The hydrogen bond has a wide transition zone which continuously merges with the covalent bond van der Waals and ionic interaction. Lemon juice citric acid vinegar acetic acid stomach acid and soda pop carbonic acid. In acid-base reactions this usually means dissolving in water.
Ionic strength 190 Ionization 20 Counterions 18 Ionic conductivity 15 Electrostatics 4. Citric acid is an integral part of orange juice and lemon juice. 05 and the heat of formation of this species is necessary to approximate the heat duty for the bioreactor in which citric acid is to be produced.
Acetic acid is the second simplest carboxylic acid after formic acid consisting of a methyl group attached to a carboxyl group via covalent bonding. Distinguish between ionic and covalent compounds under the following properties. Covalent Chemical Bonds I.
Choose all that apply. Group of answer choices. Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more.
Khan Academy is a nonprofit with the mission of providing a free world-class education for anyone anywhere. The pK a value is directly proportional to the standard Gibbs free energy change for the reaction. Resulting in the formation of a coordinate covalent bond.
These acids dissolve the coating of copper oxide or basic copper carbonate present on the surface of tarnished copper vessels and make them shining red-brown again. Whenever a molecule is reduced by acquiring an electron the electron e- brings with it a negative chargeIn many cases this charge is rapidly neutralized by the addition of a proton H from water so that the net effect of the reduction is to transfer an entire hydrogen atom H e-Figure 14-20B. In this work we developed a wound microenvironment self-adaptive multifunctional hydrogel based on boronic ester bonds between the QCSF and PVA which was named as QCSFP Fig.
A process that takes place when a covalent compound reacts with water to form new ions OR Breaking up of a molecule into charged components ions. There are many common acid substances. However the sustainability concerns of lithium-ion batteries LIBs and next-generation rechargeable.
Chemical compounds are pure substances that are made up of two or more elements that are chemically combined in fixed mass ratios. The Citric Acid Krebs Cycle. Ionic complementary polypeptides are chains of amino acids which can be synthesized using charged amino acid residues such as positively charged lysine K and arginine R and negatively charged glutamic acid E and aspartic acid D.
On the basis of the DFT calculations. Acids are typically ionic with a positive hydrogen ion attached to a negative anion. 1aThe boronic ester bonds in the hydrogel which contains desferrioxamine loaded gelatin microspheres email protected could react with the hyperglycemia and overexpressed ROS to.
A Enzymes are nonspecific b Enzymes speed up the rates of chemical reactions c Enzymes require a lot of energy to synthesize d Enzymes are not important in biological systems E Reactants in enzyme-catalyzed reactions are called substrates F Enzymes lower. Are you a student of engineering and looking for the largest collection of engineering quiz questions for a practice test. Experimental Data and Modeling.
Lesson 4 - Chemical Bonds II. Ionic complementary polypeptides can self-assemble into stable β-sheet nanofibers through electrostatic. You collect a dried sample of the fungus.
Lesson 3 - Chemical Bonds I. Covalent bonds within water molecules ionic bonds within water molecules hydrogen bonds between water molecules covalent bonds between water molecules. The capacity to explain the acidic or basic character of ionic species is one advantage of the Bronsted-Lowry definition of acids and bases.

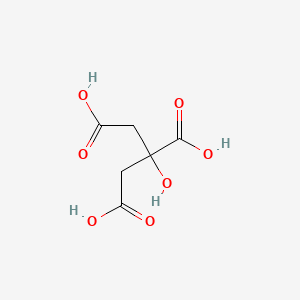
Is Citric Acid An Ionic Or Covalent Bond
Chemical Structure Of Citric Acid And Maletic Acid Download Scientific Diagram

No comments for "Citric Acid Ionic or Covalent"
Post a Comment